
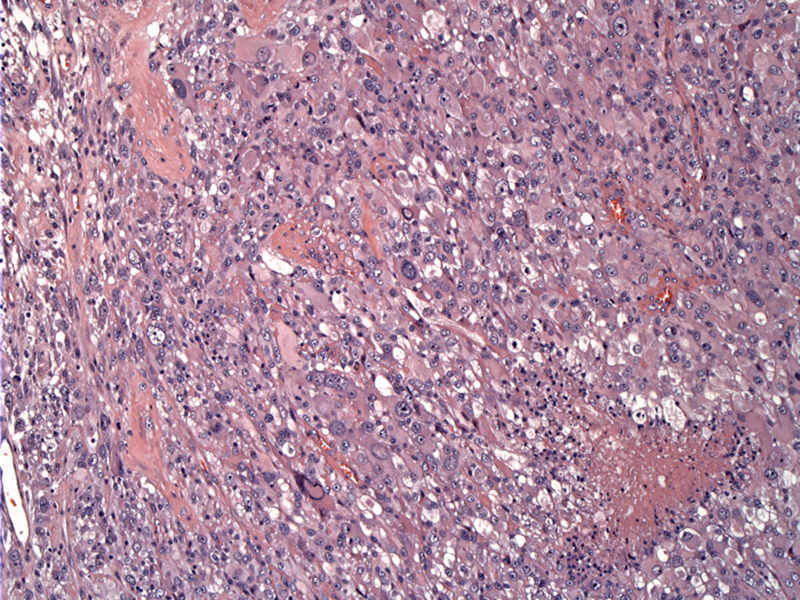
Low power shows a solid tumor proliferation comprised of variably sized malignant cells.
Astrocytic cells are simply bizarre -- extremely enlarged pleomorphic astrocytic cells can be seen with abundant eosinophilic cytoplasm.
Most of the surviving cells reside near vessels; necrosis appears to the left. Pleomorphic nuclei are multiple within a given cell.
Giant cells contain numerous nuclei, prominent nucleoli, and abundant pink cytoplasm. Smaller forms are dispersed among the larger forms.
Mitoses are seen frequently and may be atypical. The smaller cell component often harbors the higher mitotic rate in these tumors.
Ki-67 is elevated in the tumor, involving the smaller and the larger cells.
p53 mutation is also frequently seen in giant cell glioblastoma. This mutation is rare in primary glioblastomas (<10%) but are not infrequent (>65%) in secondary glioblastomas, of which approximately 90% are already present in the initial biopsy (Watanabe).
MAP-2 (microtubule associated protein) is strongly positive in a large subset of the cells. Most low and high grade diffuse astrocytomas express this protein (Wharton).
Giant cell glioblastoma is a rare variant and does afford itself a separate category in the World Health Organization classification of grade IV tumors. Due to its rarity, knowledge about this subtype is limited to small retrospective case series and case reports.
Thus far, the epidemiology and natural history does appear to differ between this tumor and classic glioblastoma, in the following ways: (1) it tends to occur in younger patients, (2)has a predilection for the temporal lobe, (3) tends to affect males more often, and (4) the prognosis seems to be better.
Giant cell GBM appears to have a blend of characteristics between primary and secondary GBM. For example, similar to secondary GBM (arising from a pre-existing glioma), it affects younger patients but like primary GBM, it arises de novo.
The molecular profile is also a hybrid of secondary and primary GBM with the following characteristics. Similar to secondary GBM, giant cell GBM tends to have TP53 mutations and lack EGFR amplification, but like primary GBM, it also tends to exhibit PTEN mutations (Fletcher, Rosai).
Histologically, the tumor is littered with prominent bizarre multinucleated giant cells. The tumor cells have abundant eosinophilic cytoplasm and frequently, pseudoinclusion in the nuclei can be seen. The tumor also exhibits a prominent reticular network in the stroma, and it has been previously named "monstrocellular sarcoma", however, GFAP positivity has confirmed its astrocytic nature (Prayson).
Imaging usually demonstrates more heterogeneous enhancement rather than the ring-enhancing lesion typical of classic GBM. This is due to the relative paucity of endothelial proliferation (Fletcher).
Children and adults may be affected. Overall it tends to occur in a slightly earlier age than the usual GBM. It tends to involve the cerebral hemispheres, mainly subcortically in the temporal and parietal lobes. Clinical symptoms are usually brief before presentation.
Several small series suggest a better prognosis for this variant i.e. 11 months median vs 8 months for other GBM (Kozak).
• Brain : Glioblastoma Multiforme
Fletcher CDM, ed. Diagnostic Histopathology of Tumors. 3rd Ed. Philadelphia, PA: Elsevier; 2007: 1666.
Kozak KR, Moody JS. Giant cell glioblastoma: a glioblastoma subtype with distinct epidemiology and superior prognosis. Neuro Oncol. 2009 Dec;11(6):833-41.
Prayson R, Kleinschmidt-Demasters BK, Cohen ML. Brain Tumors. Consultant Pathology Series New York, NY: Demos Publishing: 2010: 28-30.
Rosai, J. Rosai and Ackerman's Surgical Pathology. 9th Ed. Philadelphia, PA: Elsevier; 2004: 2508.
Watanabe, K, et al. Incidence and timing of p53 mutations during astrocytoma progression in patients with multiple biopsies. Clin. Cancer Res. 1997; 3, 523–530.
Wharton SB, Chan KK, Whittle IR. Microtubule-associated protein 2 (MAP-2) is expressed in low and high grade diffuse astrocytomas. J Clin Neurosci. 2002 Mar;9(2):165-9.