
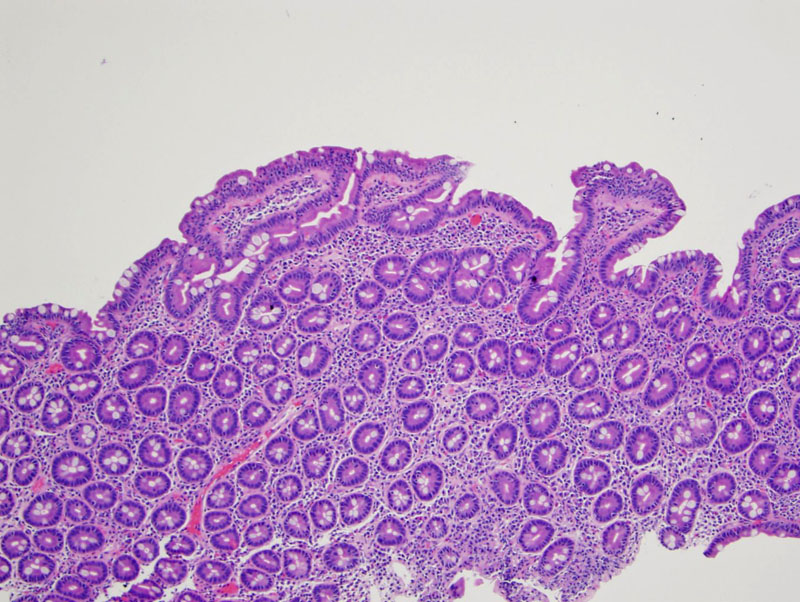
Villous architecture remains intact but blunted there is an increase in intraepithelial lymphocytes in this example of partially developed celiac sprue.
At higher power, note that the intraepithelial lymphocytes are more numerous along the tips of the villi.
At more advanged stages, there is effacement of the villi, almost looking like colonic mucosa as normal small intestinal architecture is completely lost.
Numerous lymphocytes involve the epithelium and expand the lamina propria. There is an obvious increase in cellularity of the epithelium, due to many intraepithelial lymphocytes.
Celiac disease (celiac sprue, gluten enteropathy) arises from sensitivity to gliadin, a protein found in wheat and related grains such as oat, barley and rye. It is a T-cell mediated chronic inflammatory disease with an autoimmune component and most of the affected individuals have class II HLA-DQ2 or HLA-DQ8 haplotypes. Thus, family history is extremely important in the diagnosis (Kumar).
Celiac disease is associated with a wide spectrum of gastrointestinal and extraintestinal manifestations. Histologically, there is intraepithelial lymphocytosis, expansion of the lamina propria by T cells and plasma cells, villous blunting and atrophy and crypt hyperplasia and elongation. In the early stages, one may only see subtle villi blunting and increased intraepithelial lymphocytes. A grading scheme has been developed by Marsh to communicate the severity of the disease. These biopsy findings are completely reversed if the patient goes on a gluten-free diet (Sollid, Kumar).
The risk of non-Hodgkin lymphoma is higher in patients as well as among individuals with a sibling affected with celiac disease. The increased risk of autoimmune conditions such as type I diabetes or juvenile rheumatoid arthritis is not limited to celiac disease cases but is also seen among first-degree relatives of celiac disease patients. Patients with celiac disease have a 70-fold increased risk to have microscopic colitis compared with the general population (Rubio-Tapia).
The pathophysiology is proposed as such: gliadin peptides bind to DQ2 or DQ8, which are then recognized as foreign by CD4 T cells. The CD4 T cells then stimulate the secretion of inflammatory chemokines such as interferon Y. It is not certain how CD8 T cells become involved, but they also play an important role, especially in the development of lymphoma (Kumar).
In children, the disease may present as failure to thrive. Most patients, however, are diagnosed in adulthood. Presenting symptoms include diarrhea, weight loss, flatulence and fatigue. Rarely, neurologic dysfunction can be the sole presenting feature of gluten sensitivity. Note that the patient may not necessarily be thin and malnourished, but may in fact be obese. Extraintestinal manifestations include skin rashes (dermatitis herpetiformis). Interestinly, about half of patients have an atypical presention, with symptoms such as anemia, osteoporosis, dermatitis herpetiformis, neurological problems and dental enamel hypoplasia.
The diagnosis is supported by the following findings (1) signs and symptoms of malabsorption; (2) intestinal lesions on biopsy; (3) improvement of symptoms on a gluten-free diet; (4) laboratory findings such as increased IgA toward gliadin and transglutaminase; (5) having predisposing HLA-DQ2 or HLA-DQ8 haplotypes.
Celiac disease is largely concentrated in Caucasian and is extremely rare in Africans and Asians. The estimated prevalence is approximately 1:100 to 1:200 (Kumar).
Gluten-free diet, which includes avoidance of all wheat, rye, barley and spelt. Oats, maize, rice, and sorghum are generally tolerated (Sollid). Response to the proper diet usually takes a few weeks.
There are potential serious long-term consequences, such as fertility impairment, stillbirth and dysmaturity, osteoporosis, and malignancy (most often lymphoma) (van der Windt). Strict adherence to a gluten-free diet typically resolves symptoms and can prevent long-term consequences. Some patients, especially those diagnosed above the age of 50, do not respond to a gluten-free diet These patients are diagnosed as having refractory celiac disease when clinical and histological symptoms persist or recur after a former good response to a strict gluten-free diet and despite strict adherence to the diet for more than 12 months.
Celiac disease is an autoimmune-mediated chronic enteropathy triggered by gluten proteins from wheat, barley and rye resulting in malabsorption
A diagnosis is made when the following are present: typical small-intestinal histopathological abnormalities, and clinical remission on a strict gluten-free diet with relief of symptoms within weeks
Serological tests for the diagnosis include IgA autoantibodies against the endomysium of connective tissue (EMA) and against tissue transglutaminase (TGA)
• Small Intestines : Bacterial Overgrowth
Kumar V, Abbas AK, Fausto N. Robbins and Cotran Pathologic Basis of Disease. 7th Ed. Philadelphia, PA: Elsevier; 2005: 843-4.
Rubio-Tapia A, Murray JA. Celiac disease. Curr Opin Gastroenterol. 2010 Mar;26(2):116-22.
Sollid LM, Lundin K. Diagnosis and treatment of celiac disease. Nature. 2009 Jan;2(1):3-7.
van der Windt DA, Jellema P, Mulder CJ, Kneepkens CM, van der Horst HE. Diagnostic testing for celiac disease among patients with abdominal symptoms: a systematic review.JAMA. 2010 May 5;303(17):1738-46.
