
Case 1: The adenoma is surrounded by a capsule. Small to medium sized vessels are usually present within the capsule. By definition, a follicular adenoma does not exhibit capsular or vascular invasion.
Neoplastic cells form tightly packed follicles, trabeculae or solid sheets. Rare papillary formations may be found (not seen here).
The neoplastic cells have round nuclei with inconspicuous nucleoli and ample cytoplasm. Although some nuclei may be pale and somewhat pleomorphic, the nuclei do not possess the features seen in papillary thyroid carcinoma.
Case 2: The capsule is thin but does delimit the tumor from normal thyroid parenchyma (lower image).
Clear cell variant -- the tumor cells in this example exhibit abundant clear cytoplasm. In some instances, you may need to differentiate from metastatic renal cell carcinoma, which would be thyroglobulin negative and TTF1 negative.
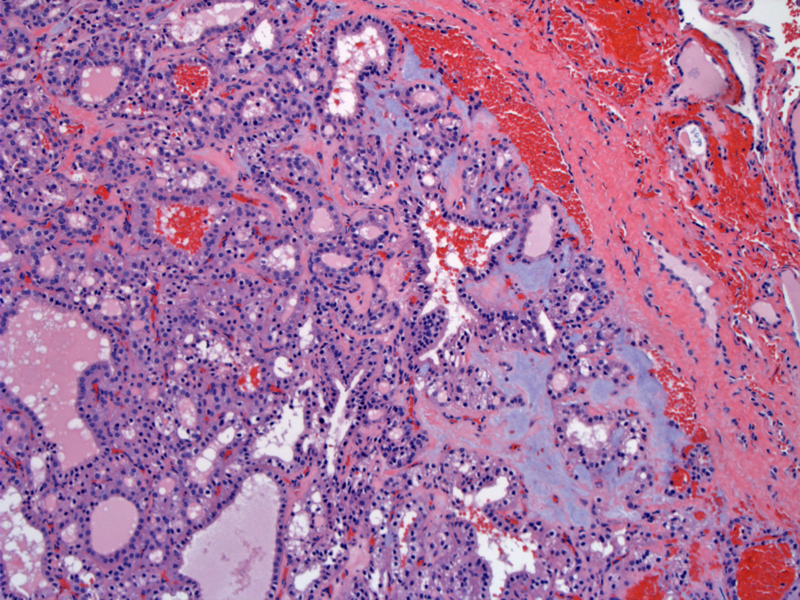
Case 3: A follicular adenoma with fairly good sized follicles is seen in the upper image. Make sure that there is no capsular invasion and that the vessels (seen within the capsules) do not contain tumor.
Secondary degenerative changes such as stromal sclerosis (seen here), hemorrhage, cyst formation, calcifications are common.
Follicular adenomas and follicular carcinomas are benign and malignant (respectively) encapsulated neoplasms that show follicular differentiation but do not exhibit the nuclear features seen in papillary thyroid carcinomas (DeLellis, Fletcher). Only the presence of capsular and/or vascular invasion distinguishes between the former from the latter, thus, the capsule should be sampled thoroughly and perhaps entirely.
Follicular adenomas account for the majority of benign neoplasms in the thyroid. Grossly, these are solitary masses (mean size of 3.0 cm) surrounded by a capsule with a homogeneous cut surface. Secondary degenerative changes are common e.g. cystic change, calcifications, infarctions (Thompson). The capsule tends to be much thicker in follicular carcinomas.
Histologically and on FNA, follicular adenomas may be virtually indistinguishable from a dominant adenomatoid (colloid) nodule. It may not really be necessary to make this distinction since both are benign.
Variants of follicular neoplasms include hyalinizing trabecular adenoma (although some authors prefer to classify this tumor as a variant of papillary thyroid carcinoma), clear cell, signet ring, mucinous, oncocytic (a.k.a. Hurthle cell) adenoma/carcinomas (Fletcher).
In terms of cytogenetic lesions, up to 1/3 of follicular adenomas and 2/3 of follicular carcinomas exhibit the PAX8/PPARgamma1 translocation. This t(2;3)(q13;p25) translocation fuses PAX8, a thyroid transcription factor, to PPARgamma1, a peroxisome-activate receptor (Kroll). Note that the follicular variant of papillary carcinoma also exhibit this translocation. These two related entities (follicular neoplasms and the follicular variant of PTC) share this genetic lesion as well as mutations in RAS.
In a study by Kroll (2000), 5 of 8 follicular carcinomas exhibited the translocation, which was not seen in 20 follicular adenomas, 10 papillary carcinomas or 10 multinodular hyperplasias.
Unlike conventional PTC, follicular neoplasms do not exhibit the RET/PTC translocation or BRAF mutations (Fletcher).
For both follicular adenomas and carcinomas, there is a female predominance (F:M ratio of 2:1) with a peak age in the 5th and 6th decades. The neoplasm presents as a painless solitary thyroid mass. The peak incidence in oncocytic (Hurthle) carcinoma is a decade later than conventional follicular carcinoma.
The incidence of follicular carcinoma is higher in iodine-deficient regions. With the widespread supplementation of iodine, the incidence of follicular neoplasms have decreased while the incidence of papillary thyroid carcinoma has increased (DeLellis). Also, follicular neoplasms are vanishingly rare in children, while papillary thyroid carcinoma (though not common before age 15) are the most common thyroid cancers in children (DeLellis).
Follicular adenomas are benign and excision is curative. The prognosis for follicular carcinoma is quite good as well, with >90% surviving past 20 years (Thompson). The oncocytic variant of follicular carcinoma has poorer prognosis as it is a more aggressive tumor (DeLellis, Fletcher).
To separate an adenoma from a carcinoma, you should look for capsular or vascular invasion. Note, however, that previous FNA may cause defects in the capsule, but usually, there should be a visible needle tract accompanied by hemosiderin laden macrophages and reactive changes (e.g. fibrosis, endothelial hyperplasia)(Thompson).
→The presence of capsular or vascular invasion distinguishes follicular adenoma from follicular carcinoma.
→The two cytogenetic lesions seen in follicular neoplasms are RAS mutations and the PAX8/PPARy translocation.
DeLllis RA, Lloyd RV, Heitz PU, Eng C. Tumors of Endocrine Organs: WHO Classification of Tumours. Lyon; IARC Press; 2004: 67-72.
Fletcher CDM, ed. Diagnostic Histopathology of Tumors. 3rd Ed. Philadelphia, PA: Elsevier; 2007: 1015-1026.
Kroll TG, Sarraf P, Pecciarini L et al. -PPARgamma1 fusion oncogene in human thyroid carcinoma [corrected]. Science. 2000 Aug 25;289(5483):1357-60.
Thompson LDR. Endocrine Pathology: Foundations in Diagnostic Pathology. Philadelphia, PA: Elsevier; 2006: 51-64, 101-8.
