System: CNS: Cerebellum: Neoplastic: Pilocytic Astrocytoma

System: CNS: Cerebellum: Neoplastic: Pilocytic Astrocytoma


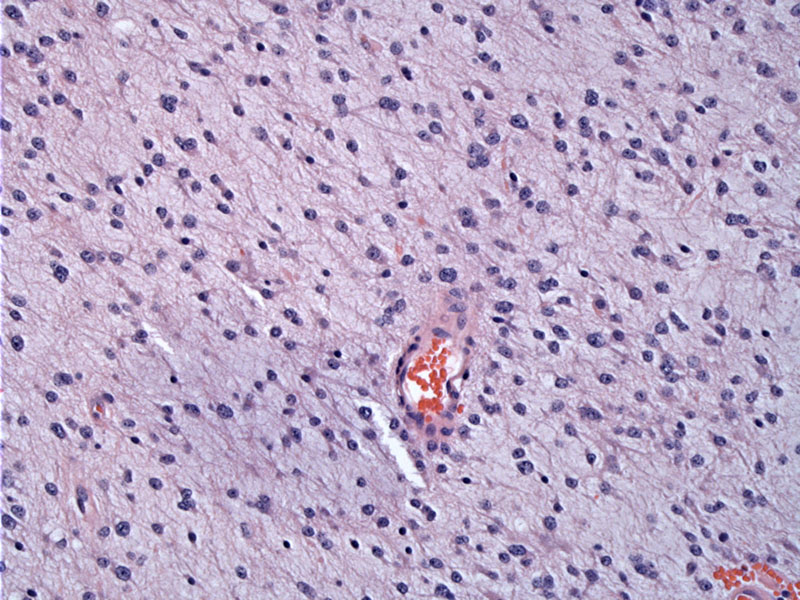
Piloid cells form a diffuse sheet. In this field, one cannot distinguish between pilocytic astrocytoma and diffuse fibrillary astrocytoma.
Perivascular aggregate of piloid cells can be appreciated and is characteristic of this tumor.
GFAP highlights the fibrillary meshwork of piloid cells.
Microcystic areas are displayed here.
Pilocytic astrocytomas, a variant of astrocytomas, are relatively benign tumors that arise mostly in the cerebellum. Other less common locations include the floor and walls of the third ventricle and optic nerves. Note that these are all midline structures. Rarely, the tumor can arise in the cerebral hemispheres or the spinal cord (Fletcher, Kumar).
Grossly, the lesion is often cystic with a mural nodule in the cystic wall. The tumor border is usually well-delineated and unlike diffuse fibrillary astrocytomas, pilocytic astrocytomas do not diffuse infiltrate into the surrounding normal parenchyma. There is a tendency to involve the leptomeninges, optic nerves and optic chiasm; however, this does not indicate malignant behavior or predict CSF dissemination (Prayson).
Microscopically, a biphasic pattern is usually seen composed of the following components: (1) Piloid cells exhibit bland elognated nuclei and bipolar fibrillary pencil-like "piloid" processes. These cells form fairly compact bundles and tend to aggregate around vessels; they are strongly GFAP positive; (2) Stellate cells with shorter processes resembling astrocytes. The stellate cells often exhibit microcystic change and are weakly GFAP positive (Fletcher).
Rosenthal fibers, eosinophilic granular bodies (EGB) are common, especially in the compact piloid areas, and represent degenerate features. Note that Rosenthal fibers and EGBs are not pathognomonic for pilocytic astrocytomas, but can be found in other low-grade tumors such as gangliogliomas, peleomorphic xanthoastrocytoma and subependymomas (Prayson).
Although vascular proliferation is characteristic, this does not represent malignant transformation as it does in anaplastic astrocytic tumors (Fletcher). Furthermore, nuclear atypia, pleomorphism with multinucleation, local infiltration of the leptomeninges are features of concern. However, if increased mitotic activity is seen alongside necrosis and microvascular hyperplasia, this should arouse suspicion for anaplastic transformation (Fletcher).
In contrast to diffuse type astrocytomas, TP53 mutation and increased PDGFR-alpha expression do not play a role in pathogenesis of pilocytic astrocytomas. Approximately 30% of tumors arise in patients with NF1.
Pilomyxoid astrocytoma is a variant occuring in infants that must be recognized. It exhibits a more monomorphic pattern, a prominent myxoid stroma and lack Rosenthal fibers or eosinophilic bodies. This particular subtype have a higher recurrence rate and poorer prognosis (Fletcher, Cheng).
Typically occurs during the first two decades. It is actually the most common brain tumor between ages 5 and 19 (Fletcher).
Categorized as a WHO Grade I tumor. Prognosis is generally excellent, and malignant transformation is exceptionally rare. Prognosis also depends on the location of the tumor, and if near the hypothalamus or brain stem, total excision may not be possible and the residual tumor may slowly grow and compress critical parts of the brain (Prayson).
→Occurs most commonly in childhood or adolescence
→Predilection for the cerebellum, third ventricle and anterior optic pathway
→NF1 patients are at risk of developing bilateral "optic nerve gliomas", which are mostly pilocytic astrocytomas.
→NF1 patients are also at risk of developing cerebellar pilocytic astrocytomas.
→Histologic features include a biphasic appearance and scattered Rosenthal fibers and eosinophilic granular bodies.
Cheng L, Bostwick DG, eds. Essentials of Anatomic Pathology. 2nd Ed. Totowa, NJ: Humana Press; 2006: 370-1.
Fletcher CDM, ed. Diagnostic Histopathology of Tumors. 3rd Ed. Philadelphia, PA: Elsevier; 2007: 1667-70
Kumar V, Abbas AK, Fausto N. Robbins and Cotran Pathologic Basis of Disease. 7th Ed. Philadelphia, PA: Elsevier; 2005: 1403-4.
Prayson R, Kleinschmidt-Demasters BK, Cohen ML. Brain Tumors. Consultant Pathology Series New York, NY: Demos Publishing: 2010: 153-5.