
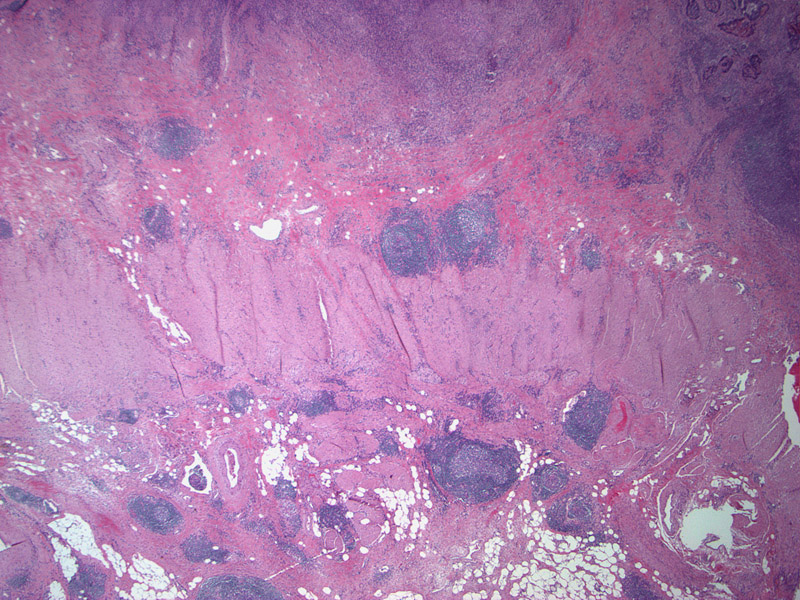
A Crohns-like reaction is seen with discrete lymphoid aggregates, some of which have germinal center. These lymphoid aggregates are usually located >=1 mm beyond advancing tumor front.
Undifferentiated carcinoma, also called medullary carcinoma, consists of a sheet of malignant cells with no gland formation -- this histology is almost pathognomonic of MSI, with published rates of MSI-H from 86% to 100% (Gafa, Lanza).
A striking degree of intraepithelial lymphocytosis can be seen here. Tumor-infiltrating lymphocytes are a fairly sensitive marker of MSI colorectal carcinomas.
Of course, the histology of MSI tumors also consists of conventional gland-forming adenocarcinoma. Here is an example of a documented MSI tumor with well-differentiated areas. Note the high mitotic activity.
MSI tumors have been described as having distinctive clinicopathologic characteristic such as a preponderance for the right colon, arising more frequently in women, and often showing prominent mucinous differentiation, as seen in the lower half of this image. Note that histologies may differ depending on whether the mutated gene is hMLH1 or hMSH2. Mucinous carcinomas are most common in hMLH1 tumors and less so in hMSH2 tumors (Wright).
The "microsatellite instability pathway" of colorectal carcinogenesis involves mutations of DNA mismatch repair genes. Mutation of these repair genes allows errors in replication of microsatellites (repeated sequences in DNA) to be uncorrected -- thus subsequent daughter cells will have microsatellites of differing lengths, so-called "microsatellite instability".
Most of these microsatellites are situated in non-coding regions where mutations are usually harmless. However, some microsatellites are located in the promoter or coding regions of growth-regulating genes. Changes in DNA length in these instances will cause frameshift mutations, eventually leading to inactivation or alterations of tumor suppressor and growth-regulating genes.1 In sporadic cases of MSI colorectal cancers, methylation of the promoter region of MLH1 (one of the DNA mismatch repair genes) results in silencing of the gene.3
A note of terminology: The terms applied to these tumors can be quite confusing. MMR (mismatch repair), MSI (microsatellite instability), Lynch syndrome, HNPCC (hereditary nonpolyposis colorectal carcinoma) all refer to carcinogenesis involving mutations or silencing of these 'proof-reader' or mismatch-repair genes. Got it?
10-15% of sporadic colorectal cancers develop via this pathway as well as tumors in the HNPCC (Hereditary Nonpolyposis Colorectal Cancer) syndrome. Patients with HNPCC (Lynch Syndrome) have a tendency to develop cancer of the colon and extra-intestinal locations such as the endometrium, ovary, stomach and brain. HNPCC patients inherit one mutated copy of a DNA repair gene from a parent ("first hit") and for unclear reasons, cells in the colon, stomach and endometrium are prone to acquiring a second somatic mutation ("second hit"), leading to loss of heterozygosity and a mutation rate 1000 times that of an unaffected individual.1
There are five genes involved in DNA mismatch repair, and inherited mutations in any of these genes may lead to familial HNPCC. 90% of the mutations involve MSH2 and MLH1. The remainder involve MSH6, PMS1 and PMS2. Immunostaining for MLH1, MSH2, MSH6 and PMS2 may identify loss of expression of culprit mutated gene. Note that MSI tumors may NOT express CK20 or nuclear beta-catenin, which are routinely positive in conventional type colorectal adenocarcinomas.3
Tumors that develop via the mismatch repair pathway tend to exhibit the following features: (1) location in the proximal colon; (2) mucinous histology; (3) infiltration of tumor by lymphocytes.1,2
Muir-Torre Syndrome consists of cutaneous sebaceous neoplasms with colorectal cancer and is considered a variant of HNPCC.
HNPCC accounts for 1-5% of all colorectal cancers.3 Average age of diagnosis of cancer in patients with HNPCC is 45. Synchronous and metachronous colon cancers may be seen in up to 25% of HNPCC patients.2 HNPCC patients may also present with extra-intestinal cancers (ie. ovary, stomach, brain).
It is important to identify this subset of patients with MSI colorectal carcinomas. These patients are at risk for multiple (appearing at different sites) and metachronous (appearing at different times) colorectal carcinomas. HNPCC patients are at risk for extra-intestinal tumors as well.
Area of current investigation:
Colorectal carcinomas with DNA MSI are more likely to exhibit mucinous, poorly differentiated or undifferentiated (medullary) histology -- however, some studies show that prognosis may be improved for these tumors despite having more alarming histology and a decreased response to standard chemotherapy. The reason may be the tendency for these MSI tumors to be (1) well-circumscribed and localized with less frequent lymph node involvement; (2) associated with tumor-infiltrating lymphocytes; (3) occurring in younger individuals.2,3
However, the picture becomes less clear with recent studies. Some studies show that in univariate analysis, MSI had a favorable effect on age-adjusted survival. Other studies employing multivariate analysis demonstrate that age, grade, Crohn's-like reaction and stage have proven independent predictors of survival, but MSI status has not (Kakar).
Another study compared MSI tumors to matched controls of MSS (microsatellite stable) tumors -- the study did not find a survival advantage for either untreated MSI tumors nor MSI tumors treated with 5FU/mitomycin chemotherapy (Storojeva, 2005).
Thus, it appears that further investigation is necessary before we truly understand the prognostic implications of having an MSI tumor, and their response to adjuvant chemotherapy.
• Colon : Adenocarcinoma, Mucinous Signet Ring Type
• Colon : Adenocarcinoma, Conventional Type
• Endometrium : Endometrial Carcinoma, Mismatch Repair Related
• Colon : Mucinous Adenocarcinoma
• Colon : Adenocarcinoma, Mucinous Signet Ring Type
1 Kumar V, Abbas AK, Fausto N. Robbins and Cotran Pathologic Basis of Disease. 7th Ed. Philadelphia, PA: Elsevier; 2005: 862,864.
2 Iacobuzio-Donahue CA, Montgomery EA. Gastrointestinal and Liver Pathology: Foundations in Diagnostic Pathology. Philadelphia, PA: Elsevier; 2005: 382-9.
3 Fletcher CDM, ed. Diagnostic Histopathology of Tumors. 3rd Ed. Philadelphia, PA: Elsevier; 2007: 403.
Chapusot C, Martin L, Mungra N et al. Sporadic colorectal cancers with defective mismatch repair display a number of specific morphological characteristics: relationship between the expression of hMLH1 and hMSH2 proteins and clinicopathological features of 273 adenocarcinomas. Histopathology. 2003 Jul;43(1):40-7.
Wright CL, Stewart ID. Histopathology and mismatch repair status of 458 consecutive colorectal carcinomas. Am J Surg Pathol. 2003 Nov;27(11):1393-406.
Smyrk TC, Watson P, Kaul K, et al. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer. 2001; 91:2417–2422.
Gafa R, Maestri I, Matteuzzi M, et al. Sporadic colorectal adenocarcinomas with high-frequency microsatellite instability. Cancer. 2000; 89:2025–2037.
Lanza G, Gafa R, Matteuzzi M, et al. Medullary-type poorly differentiated adenocarcinoma of the large bowel: a distinct clinicopathologic entity characterized by microsatellite instability and improved survival. J Clin Oncol. 1999; 17:2429–2438.
Kakar S, Aksoy S, Burgart LJ, Smyrk TC. Mucinous carcinoma of the colon: correlation of loss of mismatch repair enzymes with clinicopathologic features and survival. Mod Pathol. 2004 Jun;17(6):696-700.
***Histology images courtesy of Dr. Appelman, Dept of Pathology at University of Michigan, Ann Arbor MI.