
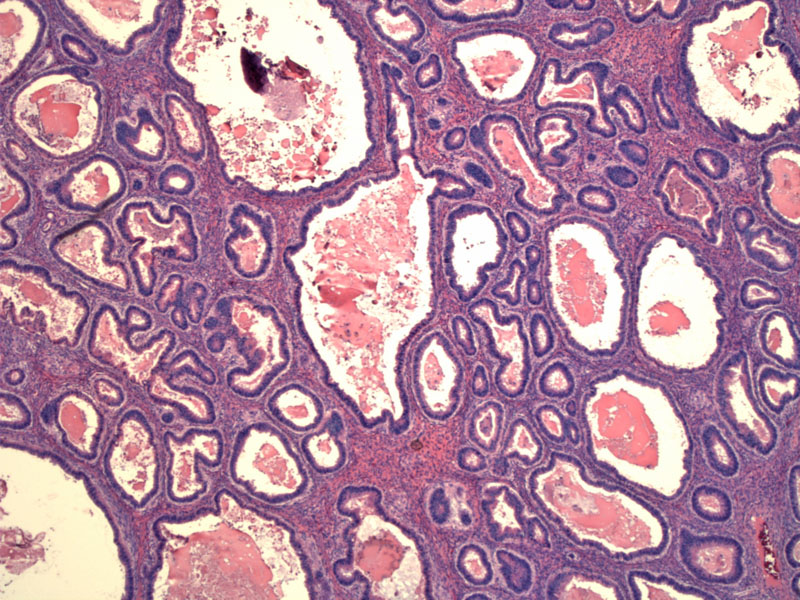
Expansile growth of closely-packed malignant glands replace large areas of the ovarian stroma. The area must exceed 10mm2 and be at least 3mm in each of two linear dimensions to qualify as stromal invasion.
At higher power, necrotic debris and eosinophilic material can be seen in the center of glands.
A different case also demonstrating crowded expansile glandular growth with replacement of normal ovarian parenchyma. The glands in this tumor have a more complex lining, exhibiting papillary formation and undulations.
The cells lining the glands are enlarged with mucin containing eosinophilic cytoplasm. Note that in just one field, there is pronounced variation in nuclear atypia. The glands in the upper image are benign-appearing and the glands in the lower image are markedly atypical.
Stratification and piling up of malignant cells are superimposed on cribriform glandular growth.
Cytokeratin 7 stains the majority of this tumor. For mucinous tumors for which the morphologic differential diagnosis is between ovarian versus metastatic lower intestinal tract carcinoma, the presence of diffuse CK7 expression coupled with focal expression of CK20 favors ovarian origin. Only focal CK7 expression in conjunction with diffuse expression of CK20 favors lower gastrointestinal tract tumors (ie. colorectal carcinoma). However, neither CK7/CK20 expression profiles nor staining distribution helps distinguish primary ovarian mucinous tumors from metastatic upper gastrointestinal tract (pancreatic, gallbladder/biliary tract, and gastric) or endocervical carcinomas (Vang).
While some ovarian mucinous tumors exhibit the characteristic CK7+/CK20- profile of the ovarian serous tumors, the majority coexpress CK20 to some degree (Vang). This particular tumor shows variable CK20, with some negative areas and some scattered positive cells in this particular field.
Other areas of the same tumor show even stronger keratin 20 staining, again demonstrating the point that IHC staining can be variable. If the neoplastic glands show largely negative staining with focal areas of strong CK20 positivity, this tumor still fits the CK7+/CK20- pattern for an ovarian primary.
A mucinous carcinoma of the ovary is usually large (15-20 cm) in diameter and unilateral. Surface ovarian involvement is not common, although due to its large size, the capsule may rupture leading to mucin extravasation and adhesions. As a general rule of thumb, bilateral and small (less than 10 cm ) mucinous tumors are more likely to be metastatic whereas large and unilateral tumors are more likely to be primary to the ovary.1
Invasive mucinous carcinomas exhibit two general patterns of invasion: (1) confluent or expansile pattern is exhibited by back-to-back glands or papillae with scant intervening stroma; cribriforming may be also be present; (2) destructive stromal invasive pattern occurs when irregular single cells or nests of malignant cells infiltrate the stroma. The pattern of invasion should be noted on the pathology report as the risk of recurrence is higher in the infiltrate pattern.1,2
Stromal invasion must exceed 3 mm in linear extent or involve an area greater than 10mm2 to be classifed as a carcinoma. Otherwise the tumor would be classified as a borderline tumor with microinvasion, which carries a favorable prognosis.
In the vast majority of cases, intestinal-type glands predominate in mucinous carcinomas. The glands lined by columnar cells with eosinophilic cytoplasm. Stratification is common, the nuclei are enlarged, pleomorphic, vesicular with prominent nucleoli. Goblet cells may be present. A component of benign or borderline mucinous neoplasm is often seen admixed with carcinomatous areas.
Some mucinous carcinomas exhibit endocervical-type glands, however, these neoplasm are rare and have not been well-described. The immunophenotype would be similar to its borderline counterpart (CK7+, CD20-, ER+ and PR+). These tumors may be less aggressive than the intestinal-type.1
Presentation is similar to borderline mucinous tumors. Patients may be asymptomatic or present with abdominal enlargement and pain. CA-125 may not be markedly elevated (unlike serous carcinomas).
Surgical staging is as for epithelial ovarian cancers, including total hysterectomy, bilateral salpingo-oophorectomy, omentectomy, pelvic lymphadenectomy, washings, and peritoneal biopsies of the pelvis, abdomen, and diaphragm. A secondary goal of staging surgery is optimal cytoreduction, leaving no disease >1cm. Adjunctive chemotherapy is with a platinum and taxane combination regimen, with recent research looking into heated intraperitoneal delivery methods.
Mucinous invasive carcinomas with an expansile growth pattern are generally stage I and have an excellent prognosis while those with an infiltrative growth pattern are more aggressive, accounting for almost all high-stage mucinous tumors, and responsible for most deaths caused by tumor (Hart). Patients often ultimately succumb to small bowel obstruction.
• Ovary : Mucinous Borderline Tumor, Endocervical-type (Case 2)
• Ovary : Mucinous Cystadenocarcinoma (Infiltrative Type)
• Ovary : Colonic Adenocarcinoma
1 Fletcher CDM, ed. Diagnostic Histopathology of Tumors. 3rd Ed. Philadelphia, PA: Elsevier; 2007: 577-8.
2 Nucci MR, Oliva Esther. Gynecologic Pathology: Foundations in Diagnostic Pathology. Philadelphia, PA: Elsevier: 2009: 421-3.
Hart WR. Mucinous tumors of the ovary: a review. Int J Gynecol Pathol 2005 Jan;24(1):4-25.
Shimada M, et al. Clinicopathological characteristics of mucinous adenocarcinoma of the ovary. Gynecol Oncol 2009 Jun;113(3):331-4.
Vang R, Gown AM, Barry TS et al. Cytokeratins 7 and 20 in primary and secondary mucinous tumors of the ovary: analysis of coordinate immunohistochemical expression profiles and staining distribution in 179 cases.Am J Surg Pathol 2006 Sep;30(9):1130-9.
Vang R, Ronnett BM. Distinction of Primary Ovarian Mucinous Tumors and Mucinous Tumors Metastatic to the Ovary. Pathology Case Reviews 2006;11:18-30.