
Case 1: Invasive glands are seen in the cervical stroma. Malignant endocervical cells contain abundant mucin and basally oriented nuclei. Desmoplasia is likely present but is obscured by the lymphocytic reaction.
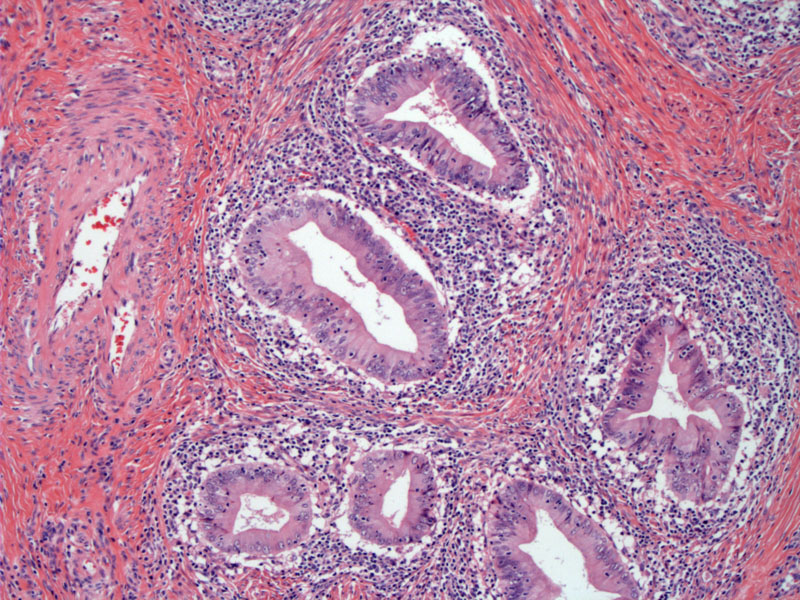
Case 2: A different case demonstrates crowded glands with complex architecture. The nuclei of these glandular cells are markedly atypical, overlapping, hyperchromatic with high N/C ratio.
The glands heres have an appearance of endometrioid adenocarcinoma (versus endocervical adenocarcinoma) -- this distinction is subjective and there is no difference in prognosis. Endometrioid adenocarcinoma of the cervix, however, must be distinguished from endometrioid adenocarcinoma of the endometrium.
Case 3: This case of cervical adenocarcinoma exhibits an unusual appearance. The malignant cells are forming diffuse sheets and poorly formed glands containing eosinophilic secretions. The nuclei are pleomorphic with prominent nucleoli.
Another area of this tumor demonstrates ragged islands of glandular cells infiltrating through cervical stroma, eliciting an inflammatory response.
Yet another area shows cord-like infiltrating glands and cystic glands of varying caliber. Note the marked atypia of the glandular cells.
Case 4: This is an example of an adenocarcinoma fragment obtained from a endocervical curettage. It was difficult to determine if this represented an endocervical or endometrial primary carcinoma.
This following set of stains demonstrate the difficulties in interpreting IHC panels. For example, endocervical neoplasias tend to be CEA positive. In this instance, CEA immunostaining was entirely negative, thus favoring an ENDOMETRIAL primary.
However, there was strong staining for p16 which favors an ENDOCERVICAL lesion.
Resection yielded a deeply invasive high grade papillary serous adenocarcinoma arising from the lower uterine segment (endometrium). This demonstrates one pitfall of immunostaining in this setting -- namely p16 positivity can be seen in high grade endometrial adenocarcinomas as well as endocervical primaries.
IHC Chart: A recent study of 201 serous endometrial adenocarcinomas, endometrioid endometrial adenocarcinomas and endocervical adenocarcinomas revealed that p16 can distinguish between endometrioid endometrial adenocarcinomas (negative p16) versus endocervical adenocarcinomas (positive p16). However, serous (high-grade) endometrial adenocarcinomas are also p16 positive. Therefore, one must employ other means to distinguish between the two entities. Namely, p53 is positive and CEA is negative in endometrial adenocarcinoma, the opposite is true in endocervical adenocarcinoma. Furthermore, the presence of HPV will be detected in endocervical adenocarcinoma, but using p16 as a proxy for HPV will not be useful differentiating endocervical adenocarcinoma versus serous endometrial adenocarcinoma (Yemelyanova).
The relative incidence of invasive adenocarcinoma of the cervix has been rising, in part due to the decrease in squamous carcinoma of the cervix. Screening by Pap smear, largely responsible for detecting squamous lesions before they become invasive, is not as effective in detecting cervical adenocarcinoma.
The most common subtype of adenocarcinoma is endocervical adenocarcinoma, which resembles endocervical glands. There has been some debate on how to categorize these tumors. Some authors consider them as part of the larger category of 'mucinous adenocarcinoma', which also includes intestinal-type, minimal-deviation, and villoglandular adenocarcinomas. Others prefer to place endocervical adenocarcinoma in its own separate category.
Endocervical (usual-type) adenocarcinoma is commonly associated with high-risk HPV subtypes, particularly subtype 18, in contrast to a predominance of high-risk HPV subtype 16 in squamous carcinoma.1 Microscopically, the glands resemble endocervical epithelium, with varying amounts of mucinous or eosinophilic cytoplasm. The nuclei are enlarged, hyperchromatic and atypical (similar to adenocarcinoma in situ). Apoptotic bodies and mitotic figures are common. Architectural patterns include glandular, solid, papillary, cribriform and microcystic (which may simulate and thus must be distinguished from, microglandular hyperplasia of the cervix).
Mean age of diagnosis is approximately 55 years and risk factors are similar to those of squamous lesions. Common presentations include abnormal vaginal bleeding and occasionally, an exophytic polypoid fungating mass may be seen on exam. The cervix may be circumferentially enlarged -- so called 'barrel cervix'. The majority of lesions are located in the transformation zone, however, 15% of cases are located higher up in the endocervical canal, and thus, inapparent on colposcopy.1
Cervical adenocarcinoma can be treated either surgically or with radiation therapy. Surgical treatments can range from a simple hysterectomy for microinvasive disease to a radical hysterectomy for disease confined to the cervix to an exenteration for advanced pelvic disease. Radiation therapy is usually a combination of teletherapy and brachytherapy with chemosensitization being done with a platinum based agent.
Stage, grade and tumor size are important prognostic factors. Prognosis is similar (or slightly worse) than squamous cell carcinoma. Stage IA tumors have a 5 year survival rate of 93-100% and decreases to 83% for stage IB. 5 year survival rates for stage II, III and IV tumors are 50-59%, 13-31% and 6% respectively.1
→Adenocarcinoma of the cervix is associated with high-risk HPV types.
→Endometrioid adenocarcinoma of the cervix must be distinguished from endometrioid adenocarcinoma of the endometrium. IHC panels using p16, p53 and CEA can help you distinguish between the two entities in most cases.
• Cervix : Adenocarcinoma in situ
• Cervix : Adenocarcinoma, Villoglandular Type
1 Nucci MR, Oliva Esther. Gynecologic Pathology: Foundations in Diagnostic Pathology. Philadelphia, PA: Elsevier: 2009: 174-8.
2 Fletcher CDM, ed. Diagnostic Histopathology of Tumors. 3rd Ed. Philadelphia, PA: Elsevier; 2007: 705.
3 Sternberg SS, ed. Diagnostic Surgical Pathology.4th Ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2004: 2415-7.
4 Yemelyanova A, Ji H, Shih IM et al. Utility of p16 Expression for Distinction of Uterine Serous Carcinomas From Endometrial Endometrioid and Endocervical Adenocarcinomas: Immunohistochemical Analysis of 201 Cases. Am J Surg Pathol. 2009 Jul 20. [Epub ahead of print]